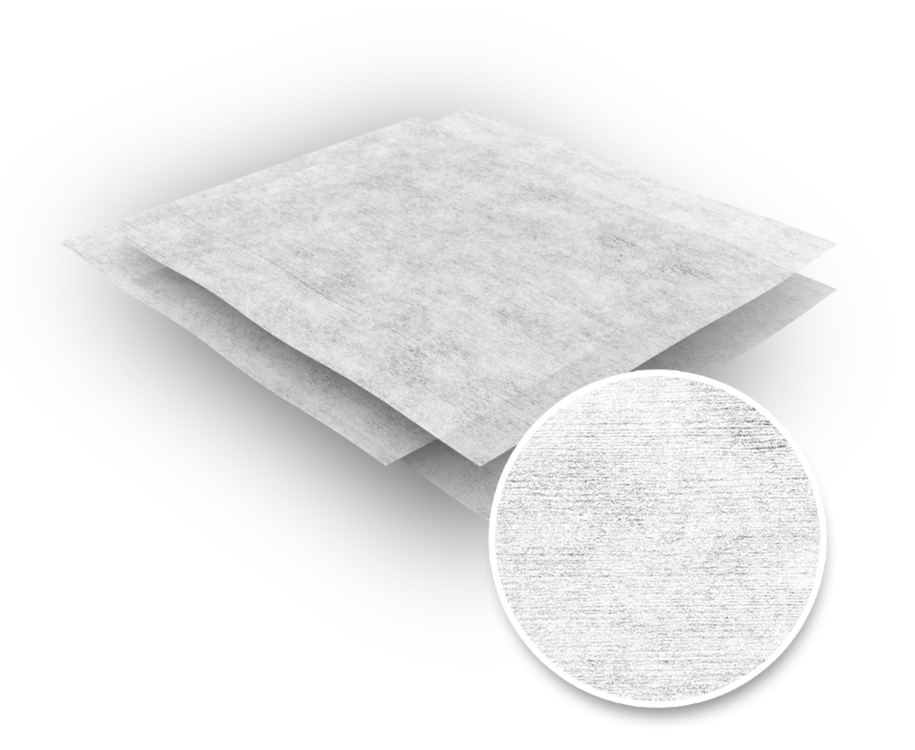
The validated sterile version of the Series 302, a polyester cellulose non-woven wipe, is versatile, both in microbiological controlled zones and in purely particulate controlled areas. Although made of a non-woven fabric, the wipe is characterized by a certain abrasion and tear resistance (even when moist).
The material of the wipe combines the different strengths of cellulose (➜ absorbency) and polyester fibre (➜ abrasion resistance) and is therefore also a happy medium in terms of costs. The hydro-bonded non-woven fabric is, in direct comparison to the series 300 and 303, cleaner.
Facts
Polyester-cellulose
Non-woven, 45% PES / 55% CEL, hydroentangled, validated sterile
Properties
- 45% polyester / 55% cellulose
- hydro-entangled non-woven fabric
- validated sterile
- low particle and fibre emission
- relatively abrasion resistant
- good absorptive capacity
- double bag packing
Advantages
- good chemical compatibility with different solvents and cleaning agents
- one of the purest polyester-cellulose non-woven wipes
- multipurpose
Applications
- all-purpose wipe partly also for more sensitive areas
- absorbency of liquids and spills
- also for the cleaning of (particulate) more critical areas (when pure polyester wipes cannot be used)
- workplace cleaning – especially for smooth surfaces
Product recommendation based on cleanroom classes
Of course, cleanroom wipes used in ISO 5 can also be used in ISO 9, but in this case the economic efficiency and usefulness should be considered.
A 1 to 1 allocation of cleanroom wipes to an air cleanliness class according to ISO 14644-1 is not possible. Recommendations can only be made on the basis of special properties relevant from a cleanroom technical point of view, such as "abrasion resistance" or "particle emission". Users can find further information on this in VDI Guideline 2083 Part 9.2.
Technical data
| Properties | Unit of measurement | Value/ | Test method/ | |
|---|---|---|---|---|
| Material | 45% PES / 55% CEL | |||
| Edge processing | cold cut | |||
| Mass per unit area | g/m2 | 68 | ||
| Thickness | mm | 0.30 | ||
|
Absorptive capacity (Ai) intrinsic (Ae) extrinsic | ml/g ml/m2 | 5.1 335 | IEST-RP-CC004.3 | |
|
NVR Non-volatile residues |
IPA based DI water based |
g/m2 g/m2 |
0.073 0.097 | IEST-RP-CC004.3 |
| Particulate residues |
0.5 – < 5.0 µm > 5.0 – ≤ 100 µm |
x 106/m2 x 106/m2 |
88 1.2 |
IEST-RP-CC004.3 Section 6.1.3 Biaxial Shake Test |
| Fibre residues | > 100 µm | fibres/cm2 | 45,550 |
IEST-RP-CC004.3 Section 6.2.2.2 |
| Ionic residues |
Sodium (Na+) Chloride (ClO2-) |
ppm ppm |
87 35 |
IEST-RP-CC004.3 Section 7.2.2.1 |
| Endotoxins | limit < 20 I.U./wipe |
pH 6.0 - 8.0 Recovery rate of the Positive Product Control (PPC)50 - 200% 94% |
corresponds < 0.50 I.U./ml 0.024 I.U./ml |
Ph. EUR. 2.6.14 LAL test, kinetic turbidimetric method (method C) |
| Organic contaminants |
silicone oil amides D-n-octylphthalate (DOP) |
n. s. n. s. n. s. |
by FTIR spectrometer Fourier transform infrared spectrometer | |
| Sterilisation | validated sterile |
Sterility Assurance Level (SAL) | 10-6 |
AAMI/ISO 11737 Sterilization of health care products – Microbiological methods – Part 1: Determination of a population of microorganisms on products |
| Size | PU/Carton | subpacked | Art. No. | |
|
6" x 6" 9" x 9" 12" x 12" 18" x 18" |
2,000 1,800 600 375 |
10 x 200 wipes (8 x 25 wipes) 12 x 150 wipes (6 x 25 wipes) 4 x 150 wipes (6 x 25 wipes) 5 x 75 wipes (3 x 25 wipes) |
57302 0606 57302 0909 57302 1212 57302 1818 | |
| n. s. = not specified n. d. = not detectable |
Note